
Molecular genetic study on plant reproduction mechnisms
1. Molecular genetic study on self-incompatibility
Self-incompatibility is an interesting trait held by Brassica. When stigmas are pollinated by pollen grains of the same plant (self-pollination), seeds are not produced, while seeds can be obtained by pollination of pollen grains from other plants (cross pollination). This is a mechanism to prevent selfing and sib crossing followed by inbreeding depression (Kitashiba and Nasrallah 2014 Breed Sci 64, 23-37). This self-incompatibility trait can be observed in many wild species and horticultural crops. It is known that the stigma cells can distinguish self-pollen grains from non-self-pollen grains, and prevent penetration of self-pollen tubes into the stigma tissue. The studies on molecular mechanism of self-incompatibility advanced most in Brassica. In Brassica, self-recognition specificity in the stigma is determined by a receptor protein encoded by SRK (S-locus receptor kinase) and that in pollen is determined by a pollen ligand protein encoded by SP11/SCR (S-locus protein 11/S-locus cysteine rich protein, SP11 hereafter). SRK and SP11 have many multiple alleles in a species, and are closely linked from each other in the S locus. Recombination between SRK and SP11 does not occur, and alleles of SRK and SP11 are inherited together to next generation. Therefore, a set of alleles of SRK and SP11 is called gS haplotypeh. SP11 protein on the surface of pollen grains is recognized by SRK on stigma surface encoded by an SRK allele of the same S haplotype, and pollen tube growth is inhibited.

In this laboratory, we discovered stigma glycoproteins (named gS glycoproteinsh) having various isoelectric points specific to S alleles (S haplotypes) and isolated them more than 30 years ago (Nishio and Hinata 1982 Genetics 100, 641-647). Then, a gene encoding this S-locus glycoprotein (SLG) has been identified in Cornell University, USA, and the SRK gene encoding the stigma recognition protein, which has a high sequence similarity to SLG, has also been isolated. Nucleotide sequence analysis of the genomic region near SRK in this laboratory enabled discovery of the SP11 gene encoding the pollen recognition protein (Suzuki et al. 1999 Genetics 153, 391-400), and the same gene named SCR was discovered in Cornell University at the same time. The structure of the S locus containing SP11, SRK, and SLG is highly variable between S haplotypes, and order and orientations of these genes and distances between them are much different (Fukai et al. 2003 Mol Gen Genet 269, 361-369; Fujimoto et al. 2006 Genetics 173, 1157-1167). This high polymorphism of the S locus is considered to be the cause of no recombination between SP11, SRK, and SLG.
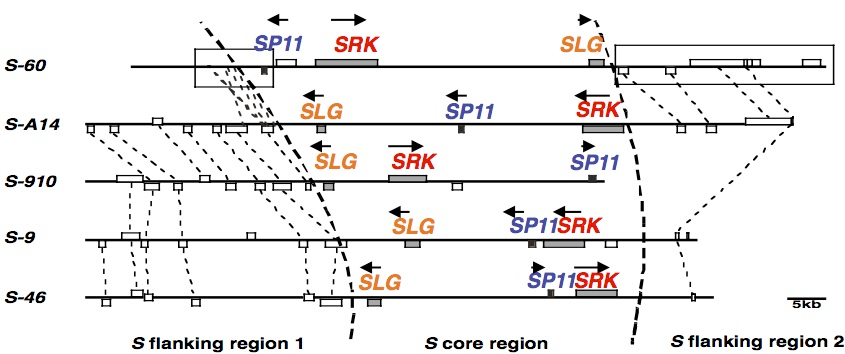
Figure. Structure of the S locus
We determined nucleotide sequences of SLG, SRK, and SP11 of many S haplotypes in Brassica rapa (Chinese cabbage, turnip), Brassica oleracea (cabbage, broccoli), and Raphanus sativus (radish), and found similar S haplotypes between different species (Sato et al. 2002 Genetics 162, 931-940). Pairs of S haplotypes having similar SLG, SRK, and SP11 sequences between B. rapa and B. oleracea and between B. rapa and R. sativus were revealed to have the same recognition specificities (Kimura et al. 2002 Plant J 29, 215-223; Sato et al. 2004 Plant Cell 16, 3230-3241). This finding suggests that new self-incompatible species in Brassica has originated by separation to a large population, not by mutations of a small number of plants. Using pairs of similar S haplotypes between different species, we identified regions determining recognition specificities in SP11 proteins, and presented a model of generation of new S haplotypes (Sato et al. 2004 Plant Cell 16, 3230-3241).
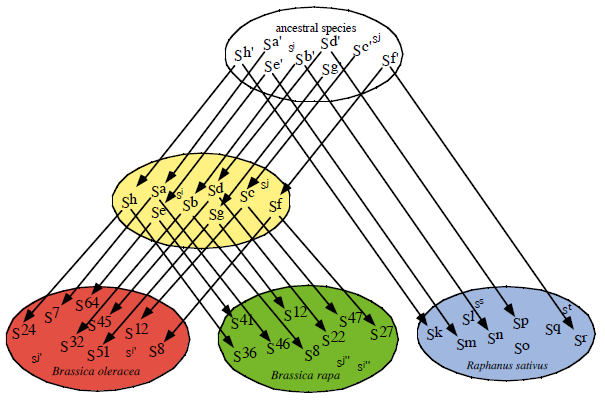
Figure. S haplotypes in a common ancestral species are shared by the present species
There are dominance-recessiveness relationships between S haplotypes. Recessiveness of an SP11 allele is known to be due to suppression of gene expression. We revealed a dominant SP11 allele is not necessary for the suppression of a recessive SP11 allele (Fujimoto et al. 2006 Plant Mol Biol 61, 577-587). Dominance and recessiveness of SRK alleles are not caused by the suppression of gene expression. Suppression of SP11 gene expression has been reported to be epigenetically controlled, but dominance-recessiveness relationships between S haplotypes are complicated, and their molecular mechanism still requires further careful investigations to be uncovered.
It is interesting that all the amphidiploid species in the triangle of U are self-compatible, while all the monogenomic species are self-incompatible. We are investigating the causes of self-compatibility of the amphidiploid species. There are several S genotypes in self-compatible Brassica napus having both the A genome from B. rapa and the C genome from B. oleracea. Self-compatibility of three S genotypes, which we investigated, was revealed to be generated by knockout mutations in the dominant S haplotypes in the A genome (Okamoto et al. 2007 Plant J 50, 391-400; Tochigi et al. 2011 Theor Appl Genet 123, 475-482).
The number of S haplotypes in a species of Brassica has been estimated to be from 50 to 100. Collection of S haplotypes in B. rapa was started ca. 40 years ago (Nishio and Hinata 1978 Jpn J Genet 53, 27-33), and followed by Nou et al. (1993 Sex Plant Reprod 6, 79-86). We have ca. 40 S haplotypes in B. rapa, now. A famous collection of S haplotypes, i.e., S tester lines, in B. oleracea was provided by Dr. Ockendon in England. Based on nucleotide sequences of these S tester lines, S haplotypes in F1 hybrid cultivars of cabbage and broccoli were identified. By selfing of F1 hybrid cultivars or crossing with the S tester lines, ca. 50 S haplotype in B. oleracea were collected. We also have ca. 20 S haplotypes in R. sativus. These are the richest collection of S haplotypes in the world. Development of these lines and propagation of seeds were performed by collaboration with Kaneko Seed Co. Ltd., Sakata Seed Co. Ltd., and Takii Seed Co. Ltd.
Self-incompatibility is used for F1 hybrid seed production, but instability of phenotype, which partly depends on S haplotypes, is one problem of F1 hybrid seed production using self-incompatibility. Therefore, a method for S haplotype identification is required. PCR-RFLP analysis of SLG has been commonly used for S haplotype identification (Nishio et al. 1996 Theor Appl Genet 92, 388-394). Since there are S haplotypes lacking SLG (Sato et al. 2002 Genetics 162, 931-940), we developed an S haplotype identification method using DNA polymorphism of SP11 (Fujimoto and Nishio 2003 Theor Appl Genet 106, 1433-1437). However, this method required some skill for DNA analysis, we improved the method to develop a simple dot-blot method (Takuno et al. 2010 Theor Appl Genet 120, 1129-1138; Oikawa et al. 2011 Mol Breed 28, 1-12).
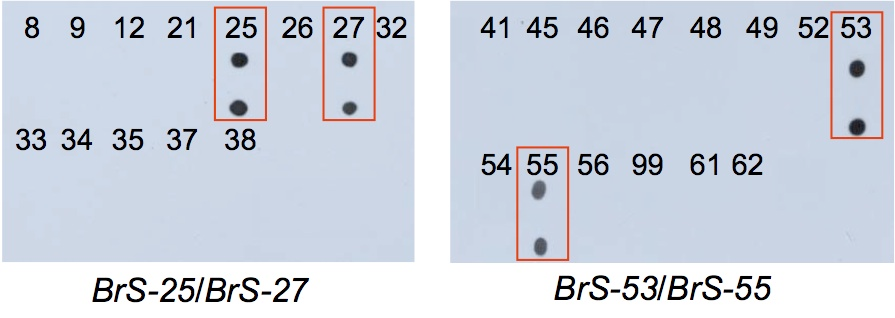
Figure. Dot-blot analysis of S genotypes in Brassica
It is considered true that specific binding of SP11 and SRK encoded by the same S haplotype triggers self-incompatibility reaction resulting in inhibition of pollen tube growth. However, downstream reaction of the binding of SP11 and SRK is not well understood or is controversial. We hypothesized the presence of M gene required for self-incompatibility by classical genetic analysis (Hinata et al. 1983 Proc Int Rapeseed Conf 1, 354-359). The M gene is considered to be responsible for the downstream reaction of the interaction of SP11 and SRK. The M gene has been reported to be MLPK encoding M-locus protein kinase, but our study using self-incompatible transgenic plants of Arabidopsis thaliana suggested the participation of MLPK in self-incompatibility to be disputable (Kitashiba et al. 2011 Proc Natl Acad Sci USA 108, 18173-18178). Although thioredoxin protein has been reported to participate in the control of SRK activity, this hypothesis was also questioned (Yamamoto and Nasrallah 2013 Plant Physiol 163, 1387-1395). In the study on the downstream mechanism of SP11-SRK interaction, we are collaborating with Professor Nasrallah in Cornell University.
2. Molecular genetic study on male sterility
Cytoplasmic male sterility (CMS) is defect of the development of male organ (anther) or male gamatephyte (pollem) resulting in the loss of fertile pollen grains and sterility that is maternally inherited. CMS is an important trait in plant breeding used for F1 hybrid seed production as well as self-incompatibility. A mitochondrial gene causing male sterility (CMS gene) and a gene restoring pollen fertility (Fertility restorer gene: Rf) participate in CMS. Alloplasmic CMS is considered to be caused by incompatibility (reproductive barrier) between mitochondria genome and nuclear genome from different species.

Figure. B. oleracea lines having Diplotaxis muralis cytoplasm. A: Sterile, B: Partial sterile, C: Fertile
Alloplasmic CMS lines of Brassica having Diplotaxis muralis cytoplasm (mur cytoplasm) were developed by interspecific hybridization and repeated backcrossing in this laboratory (Hinata and Konno 1979 Jpn J. Breeding 29: 305-311). Using these lines as materials, we investigated genes participating in male sterility. Analysis of mitochondrial genome and gene expression analysis indicated that a chimeric gene termed orf72 in mitochondria is a probable candidate of the gene responsible for male sterility (Shinada et al. 2006 Plant Cell Physiol 47, 549-553). The restorer gene for mur CMS was mapped on chromosome 1 of B. oleracea by genetic analysis using many SNP markers and a candidate gene for the Rf gene was inferred (Ashutosh et al. 2012 Mol Breed 30, 1781-1792).
Genic male sterility (GMS) due to a mutation of a nuclear gene has not been used for F1 hybrid breeding, because male sterility cannot be maintained as an inbred line. However, identification of a GMS gene enables selection of male sterile plants before planting by SNP analysis distinguishing a mutant allele from the wild type allele. We analyzed a mutated gene of a GMS mutant obtained from eKoshihikarif by mutation induction with gamma rays. The GMS gene was delimited to a 79-kb region on chromosome 9, and among the nine annotated genes in this region, a gene for flavonoid biosynthesis was found to have a frameshift mutation and was inferred to be the GMS gene (Shirasawa et al. 2013 Mol Breed 31, 805-814). A homolog of this gene in A. thaliana has been reported to be responsible for male sterility. Developing a DNA marker for detection of this mutation, we proposed an F1 hybrid seed production system using this GMS line.

Flowers and anthers of a genic male sterile mutant obtained from eKoshihikarif
3.Studies on reproductive isolation
Living organisms are generally unable to obtain progeny by crossing between different species. Even if an interspecific hybrid was obtained, that hybrid cannot produce progeny by sterility. Such barriers against interspecific hybridization are called greproductive isolationh. Reproductive isolation in plants includes interspecific incompatibility inhibiting pollen tube growth in the pistils of different species, embryo breakdown of interspecific hybrids, inability of hybrid plants to develop flowers, and hybrid sterility with no fertile pollen and/or embryo sac.
In interspecific incompatibility of Brassica species, pollen grains cannot germinate or pollen tubes cannot penetrate into a stigma tissue, and this interspecific incompatibility can be overcome by bud pollination, as observed in self-incompatibility in Brassica. Genetic analysis of interspecific incompatibility using F2 populations obtained by crossings between B. rapa lines with strong interspecific incompatibility and lines with weak interspecific incompatibility revealed that the S locus and the M locus, which are participating in self-incompatibility, have no effect on the strength of interspecific incompatibility and a gene controlling the strength of interspecific incompatibility is located on chromosome 2 (Udagawa et al. Theor Appl Genet 121, 689-696). We are narrowing down the genomic region of chromosome 2 to identify the gene controlling the strength of interspecific incompatibility.
In intergeneric hybridization between B. rapa and Raphanus sativus (radish), interspecific incompatibility can be overcome by bud pollination, but hybrid embryos break down. Depending on B. rapa lines used as a female parent, a few seeds can be obtained. Using F2 population obtained from a cross between a B. rapa line setting a few hybrid seeds and one setting no hybrid seed, we analyzed QTLs (quantitative trait loci) for hybrid seed formation ability, and found significant QTLs on chromosome 1 and chromosome 10. In these QTL regions on chromosome 1 and chromosome 10, genes homologous to Arabidopsis FIE and MSI1, respectively, which participate in seed development in Arabidopsis, were identified, and frameshift mutations were found in alleles of these B. rapa genes (Tonosaki et al. 2013 Theor Appl Genet 126, 837-846). We are going to demonstrate the function of these genes in hybrid seed formation ability by transformation of B. rapa.
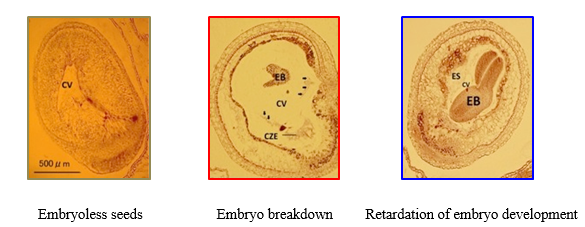
Figure. Abnormalities of embryo development observed in intergeneric hybridization
between B. rapa and R. sativus