| Name | Shinichi SATO |
|---|---|
| Position | Assistant Professor |
| Tel | +81-22-217-6198 |
| shinichi.sato.e3*tohoku.ac.jp (Please replace * with @) | |
| Research Interest | Chemical Biology, Synthetic Organic Chemistry |
| Career | Education: B.S. Meiji Pharmaceutical Univ. (2006), Graduate Scholl of Pharmaceutical Science, University of Tokyo (2011, Ph.D.) Research Experience: Postdoc at The Scripps Research Institute (2011―2012), Assistant Professor, Gakushuin Univ. Faculty of Science (2012―2014), Tokyo Institute of Technology, Chemical Resources Laboratory (2014―2016), Tokyo Institute of Technology, Institute of Innovative Research (2016―2020), Tohoku Univ. Frontier Research Institute for Interdisciplinary Sciences (2020―) |
| Research map | https://researchmap.jp/7000006665?lang=en |
| Research Projects | |
|
Elucidating and Controlling Life Phenomena with “Protein Chemical Modification”, We are developing chemical biology and drug discovery research based on “protein chemical modification technology” that forms covalent bonds between proteins and synthetic small molecular compounds. Functionalization of proteins by chemical modification requires mild reaction conditions that do not denature proteins, and the organic chemical reactions that can be used in protein functionalization research to date have been limited to only a few nucleophilic-electrophilic reactions. We are challenging the labeling and functionalization of aromatic amino acid residues in proteins by using highly reactive chemical species such as radical species and singlet oxygen. |
|
| Research Seeds | |
Protein chemical modification, a technology to introduce functionality into the chemical structure of proteins through covalent bonds, is an essential technology for the creation of biomaterials and the development of drug delivery systems using proteins. We have developed a new chemical modification reaction for proteins. This method utilizes singlet oxygen, a type of reactive oxygen species, to rapidly functionalize histidine residues. This method is expected to accelerate protein research as a new strategy to label proteins existing in local space. 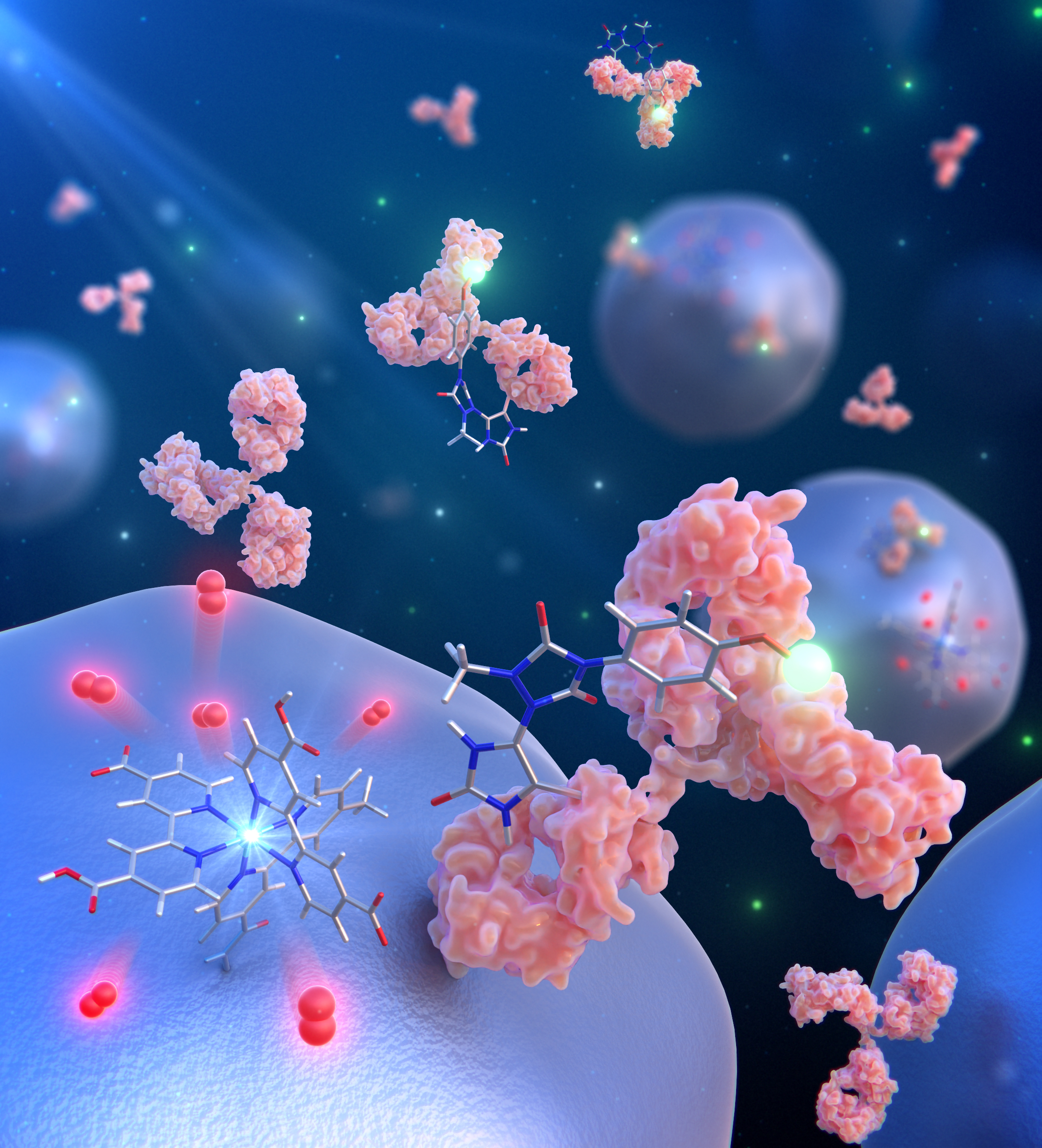 URL: http://www.tohoku.ac.jp/japanese/2021/05/press20210512-01-nano.html |
|




